Product/Service
We provide highly accurate "immature blood cell"
and unique evaluation system construction and evaluation services using these cells.
and unique evaluation system construction and evaluation services using these cells.
Our Technologies
We combine four cell-specific technologies: gene editing, differentiation induction, immortalization,
and integrated production to design specialized blood cells at will.
and integrated production to design specialized blood cells at will.
cell design technology
The "cell design technology" that comprehensively combines various factors such as gene selection, adjustment of differentiation stage, and identification of immortalization point, is a unique core competence of MyCan that is not found anywhere else.
gene editing
Genetic modification of progenitor cells can produce blood cells that reflect various genetic backgrounds.differentiation induction
We provide only high-quality hematopoietic cells in the same differentiated state with a high differentiation induction rate from iPS cells.immortalization
Immortalization prior to donation allows experiments to be performed while maintaining the original fresh state and properties. The results are similar to the normal reactions in the body, as the leukemia strain that has been immortalized from the beginning is less likely to change its properties.integrated production
Improved production efficiency enables us to produce the required amount of blood cells in large quantities and in a stable manner, and to provide them at a lower cost than in the past.Product
MiCAN Technologies is a company that produces and supplies blood cells and pseudo-viruses for research using regenerative medicine technology; it has the technology to stop myeloid lineage cells induced to differentiate from iPS cells and PBMCs before they mature and to culture large numbers of these cells. Our evaluation kits are optimized so that evaluation can be started immediately after opening the kit.Cell (biology)
We provide homogeneous and highly accurate cells with identical genes through regenerative medicine technology.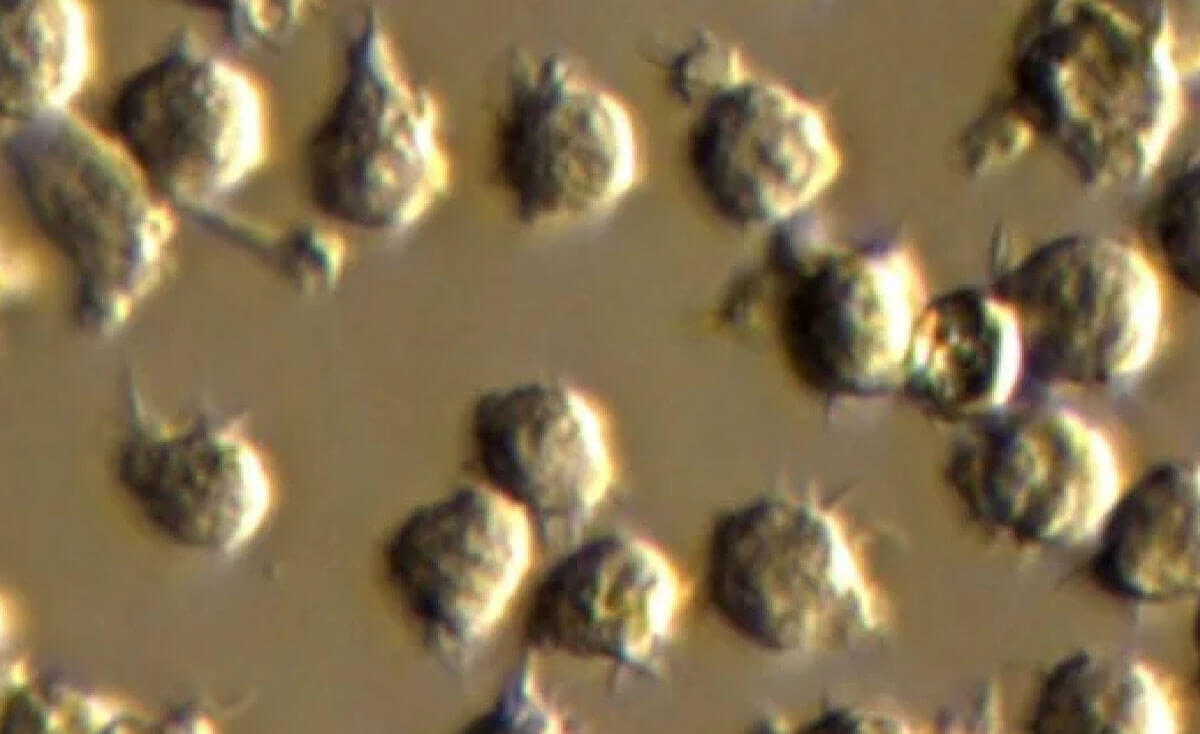
Mylc Myeloid lineage cells (Mylc cells) are myeloid lineage cells differentiated from blood and iPS cells. Myeloid lineage cells are suitable for experiments and research requiring homogeneous and highly accurate results.
Mpv Mpv cells were developed for research on Plasmodium ternate fever malaria, one of the pathogens of malaria. Using proprietary technology, undifferentiated cells are cultured in large quantities to provide a stable supply of hematopoietic cells.
Evaluation Kit
Kits for various evaluation tests can be performed immediately. Items are delivered in optimal settings.
MylcMAT : Evaluation Kit for Monocyte Activation Test (MAT) Our proprietary monocyte cell line “aMylc” offer high responsiveness, reproducibility, and stable supply for MAT applications.

Evaluation kit The Mylc cell-based cytokine (IL-6, IL-10, IL-12) evaluation kits are optimized so that highly accurate evaluations can be performed immediately after opening the kit.
Contact
Please feel free to contact us.
We will conduct a brief interview with the person in charge and propose the best solution to solve your company’s issues.
Service
We undertake various evaluations of drug candidates and functional materials using high-precision blood cell products. Blood cell cells can be freely selected and designed, and time and cost can be significantly reduced through highly accurate and efficient evaluations.Efficacy Evaluation
We can speedily evaluate the efficacy of pharmaceutical candidates and functional materials with a high degree of accuracy.
Efficacy evaluation Cellular reactions are measured with high precision. Speedy and reliable evaluation greatly accelerates drug discovery and material research.
Safety Assessment
Reduce the time and cost of various tests based on alternative laboratory animal methods.
Skin sensitization test It provides results similar to animal evaluations (LLNA) for the 10 standard compounds (P1-N10) in skin sensitization tests. The h-CLAT method does not require complicated operations and confirmations, and thus saves a great deal of time and cost.

Pyrogen test Compared to model cells (e.g., Mono-Mac-6) used in the experimental animal replacement method, Mylc, which exhibits a response similar to that of living organisms, is more sensitive and has very good stability.
Contact
Please feel free to contact us.
We will conduct a brief interview with the person in charge and propose the best solution to solve your company’s issues.
In Development
Myeloid cells are being developed for various applications, including newly developed COVID-19 related products. MylcMAT kit with immortalized myeloid cells (Mylc) (EP criteria)
Corona-SRIPs SARS-CoV-2 Omicron BQ.1.1
Corona-SRIPs SARS-CoV-2 Omicron XBB

