MylcMAT : Evaluation Kit for Monocyte Activation Test (MAT)
Developed using regenerative medicine technologies,
aMylc offers a transformative approach to MAT testing.
aMylc for MAT
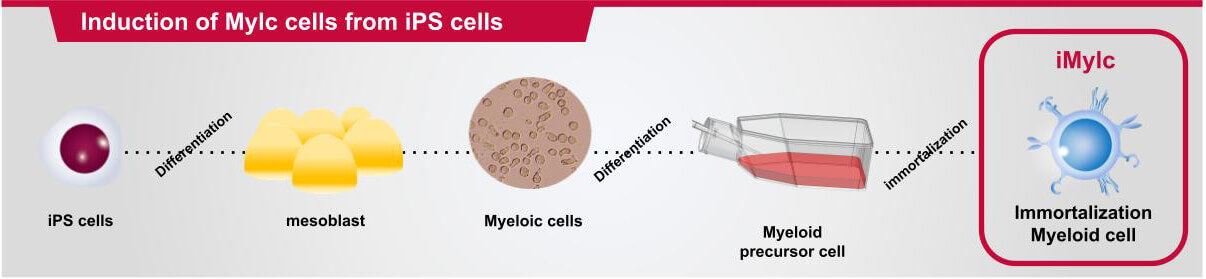
aMylc cells (Myeloid lineage cells) are immortalized myeloid cells developed by isolating monocytes from human donor peripheral blood.
aMylc has 3 main features:
1. Higher cytokine production response to stimulation compared to other cells. (Limit of Detection (LOD) < 0.025 EU/mL)
2. Production in large batches enables stable evaluation and reproducibility
3. Unique immortalized cell manufacturing technology ensures consistent production and supply.
These 3 features makes aMylc cells suitable for MAT, which requires uniform and highly accurate results.
How aMylc for MAT is produced
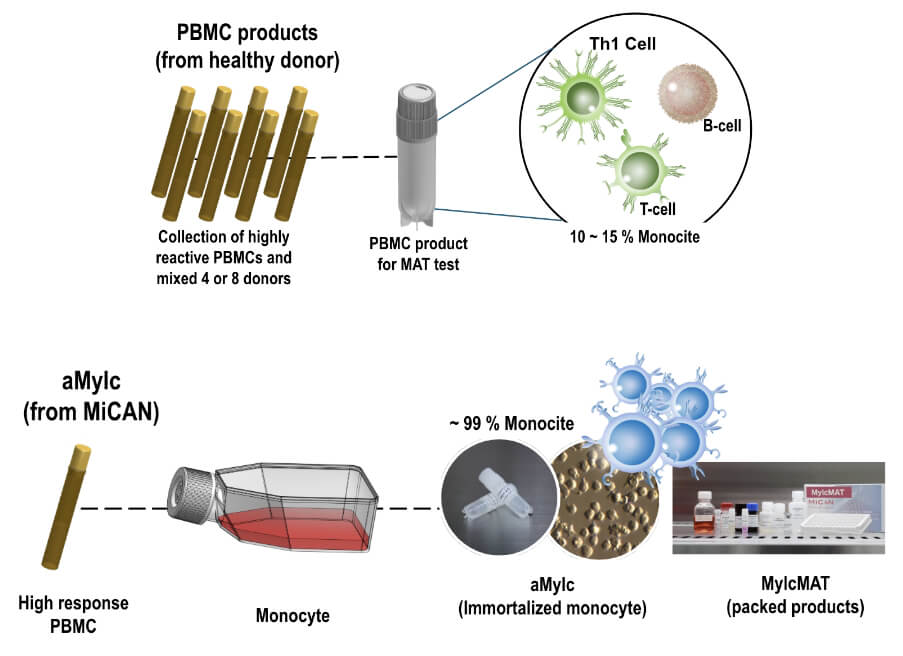
For MAT use, aMylc is produced from PBMCs that show high reactivity to microbial components.
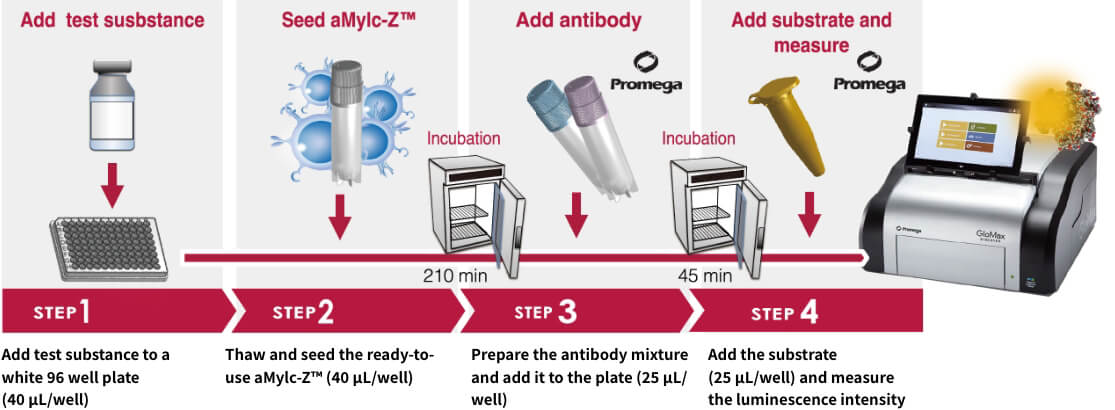
How to use MylcMAT
Why is [MylcMAT] necessary?
At MiCAN, we address these issues with our proprietary immortalized monocyte cell line (aMylc), developed by isolating monocytes from healthy donor blood and applying our regenerative medicine technology (primary cell processing, cells expansion and immortalization techniques).
aMylc replicates the reactivity of fresh blood-derived cells, while enabling large-scale, consistent production as an immortalized cell line. This allows for highly reproducible MAT testing over extended periods.
MylcMAT Kit
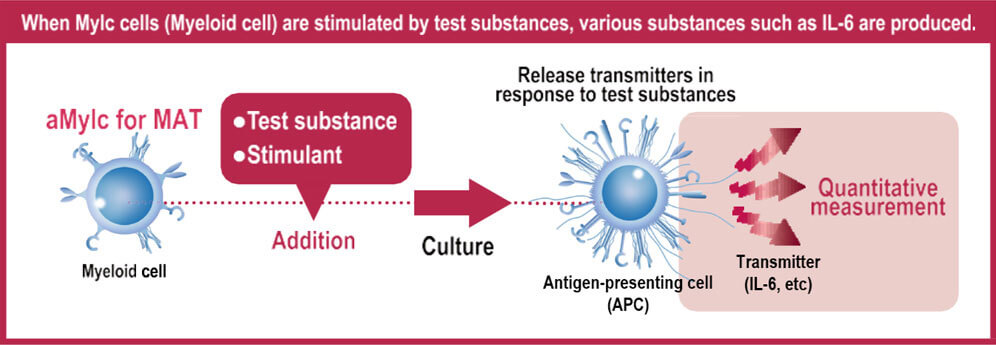
By using aMylc specifically screened for MAT, we achieved highly sensitive and consistent detection of non-endotoxin pyrogens (NEPs), surpassing the performance of conventional MAT cell preparations.
As an animal-free testing method, aMylc-based assays are free of animal-derived components(Like Fetal Bovine Serum, FBS), not only during testing, but also through the cells production and manufacturing process.
Introduction to the Next-Generation MylcMAT (MylcMAT Rapid Kit)
To overcome this, MiCAN is developing the MylcMAT Rapid Kit, designed to complete testing within a single day.
This advancement aims to facilitate the widespread implementation of MAT, aligning with the European regulatory shift towards in vitro testing methods.
Contact
Please feel free to contact us.
We will conduct a brief interview with the person in charge and propose the best solution to solve your company’s issues.

